10 000 Ppm To Percent
defexpoindia
Sep 14, 2025 · 5 min read
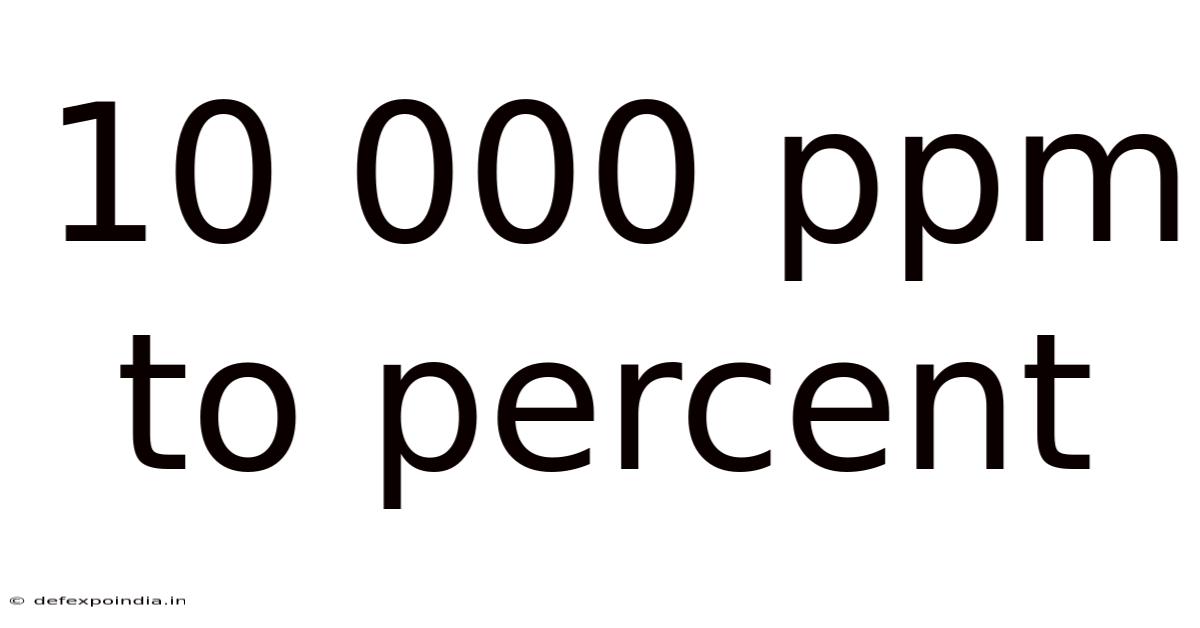
Table of Contents
Converting 10,000 ppm to Percent: A Comprehensive Guide
Parts per million (ppm) and percent (%) are both common ways to express the concentration of a substance within a mixture or solution. Understanding how to convert between these units is crucial in various fields, from chemistry and environmental science to engineering and manufacturing. This article will provide a comprehensive guide on converting 10,000 ppm to percent, explaining the underlying principles and offering practical applications. We'll also delve into the significance of this conversion and address frequently asked questions.
Understanding Parts Per Million (ppm)
Parts per million (ppm) is a dimensionless unit expressing the ratio of a solute's mass or volume to the mass or volume of the solution. It represents the number of units of a particular substance per one million units of the whole. For example, 10,000 ppm means 10,000 parts of a substance per 1,000,000 parts of the solution. This unit is particularly useful for expressing very low concentrations.
Understanding Percent (%)
Percent, denoted by the symbol %, represents a ratio expressed as a fraction of 100. For example, 10% means 10 parts out of 100 parts. It's a widely used unit for expressing concentrations, proportions, and changes.
The Conversion: 10,000 ppm to Percent
The conversion from ppm to percent is straightforward. Since percent is based on 100 parts and ppm is based on 1,000,000 parts, we simply need to scale down the ppm value. The conversion factor is 1% = 10,000 ppm. Therefore, to convert ppm to percent, we divide the ppm value by 10,000.
Calculation:
10,000 ppm / 10,000 ppm/1% = 1%
Therefore, 10,000 ppm is equal to 1%.
Detailed Explanation of the Conversion Factor
The relationship between ppm and percent can be derived from their definitions:
- Percent: Represents parts per hundred (parts/100).
- Parts per million (ppm): Represents parts per one million (parts/1,000,000).
To convert from ppm to percent, we need to find the equivalent fraction based on 100. We can set up a proportion:
x / 100 = 10,000 / 1,000,000
Solving for x:
x = (10,000 / 1,000,000) * 100
x = 1
This confirms that 10,000 ppm is equal to 1%.
Practical Applications of the Conversion
This conversion is relevant in many contexts:
-
Environmental Monitoring: Determining pollutant levels in water, soil, or air. For example, a 1% concentration of a particular pollutant might trigger environmental regulations or necessitate remediation efforts. 10,000 ppm of a specific pollutant in water, translated to 1%, immediately signals a significant contamination level.
-
Industrial Processes: Monitoring the concentration of reactants or byproducts in chemical reactions or manufacturing processes. Precise concentration control is often vital for product quality and safety. A deviation from the expected 1% (or 10,000 ppm) could indicate a malfunction or require process adjustments.
-
Medical and Pharmaceutical Applications: Measuring the concentration of drugs or other substances in the body or in pharmaceutical preparations. Accurate dosage is paramount in medicine, and understanding concentration units like ppm and percent is crucial for safe and effective treatment. For instance, certain medications might have a therapeutic range expressed in ppm, requiring conversion to percent for easier understanding and comparison.
-
Food and Beverage Industry: Controlling the concentration of additives, preservatives, or contaminants in food and beverages. Regulations often specify maximum allowable limits for specific substances, which could be expressed in either ppm or percent. Knowing the conversion is key to ensuring compliance with these standards.
-
Agriculture: Monitoring nutrient levels in soil or fertilizers. Optimal crop growth often requires precise nutrient levels, which can be expressed in ppm or percent. Farmers need to understand this conversion to manage soil fertility effectively.
Beyond the Simple Conversion: Considering Different Units
While the conversion from 10,000 ppm to 1% is straightforward when dealing with mass/mass or volume/volume ratios, the situation can be more complex when different units are involved. For instance:
-
Mass/Volume Concentration: If ppm refers to milligrams of solute per liter of solution (mg/L), then the conversion to percent requires careful consideration of units. In this case, we need to convert milligrams to grams and liters to milliliters or grams before applying the conversion factor.
-
Volume/Volume Concentration: If ppm expresses microliters of solute per liter of solution (µL/L), the conversion to percent (expressed as volume percentage, v/v) would again require unit adjustments. This conversion is particularly important in many industrial applications and analytical techniques.
Therefore, always carefully consider the units involved in the ppm value before applying the simple 10,000 ppm = 1% conversion.
Advanced Concepts and Considerations
-
Dilution Calculations: The conversion becomes more critical when dealing with dilutions. If a solution with a known concentration (e.g., 10,000 ppm or 1%) needs to be diluted to a lower concentration, the conversion helps in calculating the necessary dilution factor.
-
Mixing Solutions: When mixing solutions with different concentrations, understanding the conversion between ppm and percent facilitates the accurate calculation of the final concentration of the mixture.
-
Error Analysis: The accuracy of the conversion depends on the accuracy of the initial ppm measurement. Any uncertainty in the ppm value will propagate into the calculated percent value.
Frequently Asked Questions (FAQ)
Q: Is 10,000 ppm always equal to 1%?
A: While the simple conversion holds true for mass/mass or volume/volume ratios, it's crucial to verify the units used in the ppm value before applying this conversion. If the ppm value refers to a different unit combination (e.g., mg/L), a more complex calculation is required.
Q: How do I convert a ppm value other than 10,000 to percent?
A: Divide the ppm value by 10,000 to obtain the equivalent percentage.
Q: What are some common mistakes to avoid when converting ppm to percent?
A: The most common mistake is neglecting to check the units of the ppm value. Always confirm whether the ppm value is based on mass/mass, volume/volume, or a different unit combination.
Conclusion
Converting 10,000 ppm to percent is a fundamental calculation across diverse scientific and industrial fields. Understanding this conversion, along with the underlying principles and potential complications, is crucial for accurate interpretation and application of concentration data. Always be mindful of the units involved to ensure accurate conversions and avoid misinterpretations. By carefully considering these details, you can confidently utilize these units in various contexts and contribute to more precise calculations and analyses.
Latest Posts
Related Post
Thank you for visiting our website which covers about 10 000 Ppm To Percent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.